We ensure the design, planning, and monitoring of industrial projects, guaranteeing execution in compliance with regulatory requirements and industry best practices.
Conceptual Design : Defining requirements and establishing initial technical orientations.
Capacity Study : Analyzing production capacities and sizing equipment.
Preliminary Design (APS) & Detailed Design (APD) In-depth studies to validate technical and budget feasibility.
Preparation of Technical Specifications : Drafting technical documents for supplier consultations.



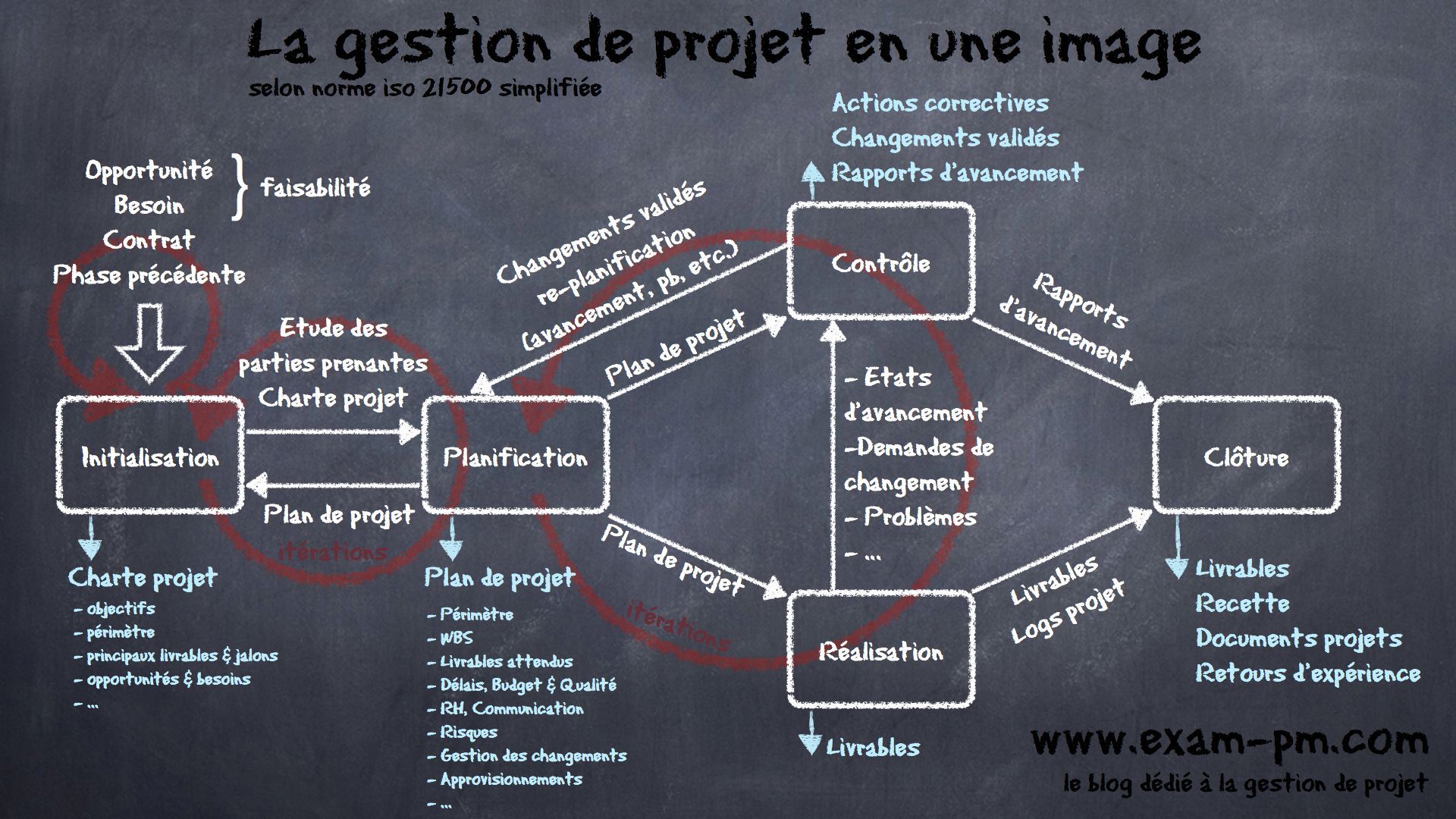

Selection of Contractors : Assistance in choosing service providers.
Supervision & Coordination : Planning and monitoring the execution of works.
Budget Management : Cost control and investment optimization.
Acceptance & Qualification : Validation of equipment and installation compliance.
Participation in FAT & SAT Inspections: :





We specialize in the sizing and design of pharmaceutical production lines tailored to various dosage forms:
Complete Workshop Design & Implementation
We conduct comprehensive diagnostics of installations to ensure compliance with current standards.
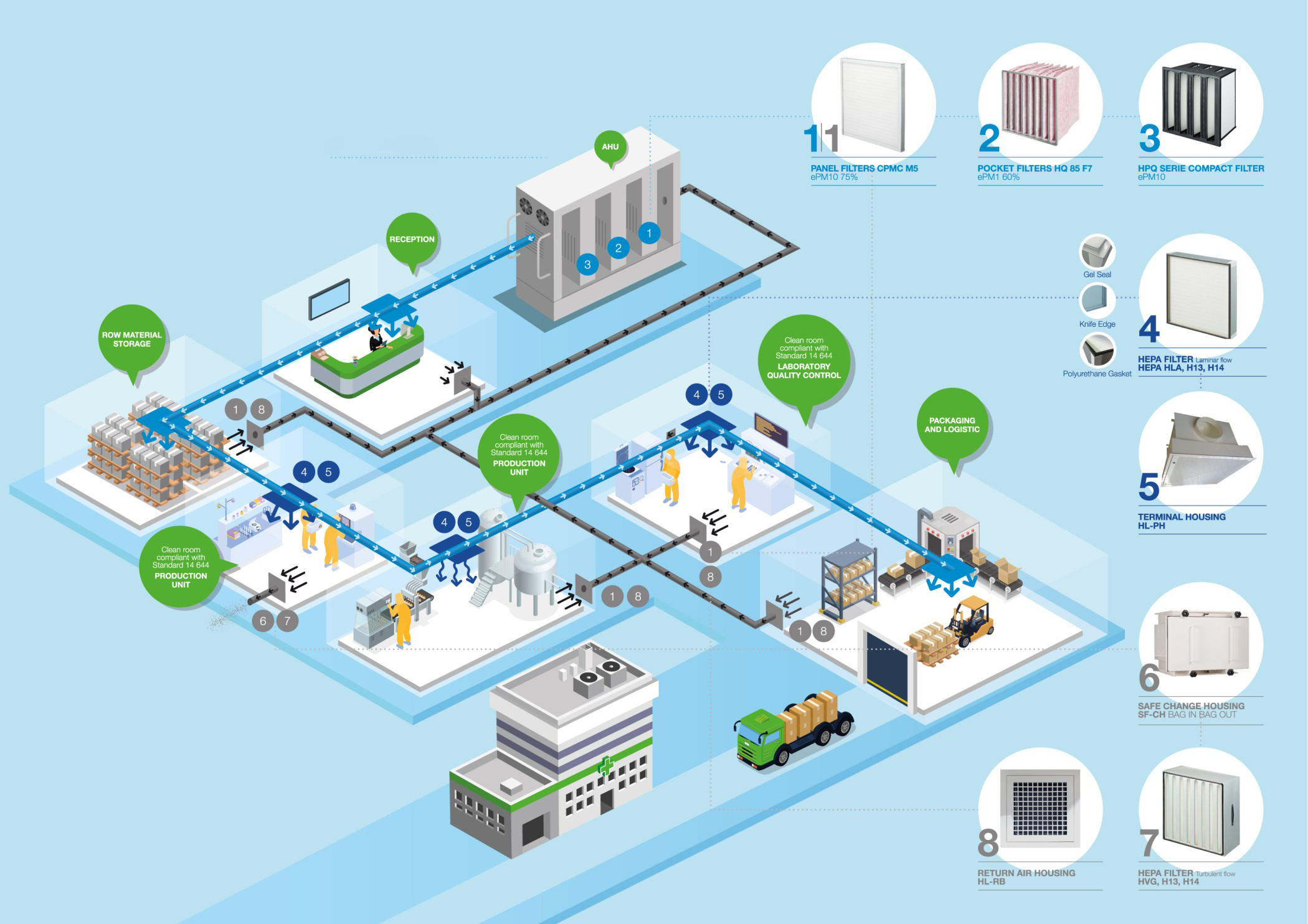





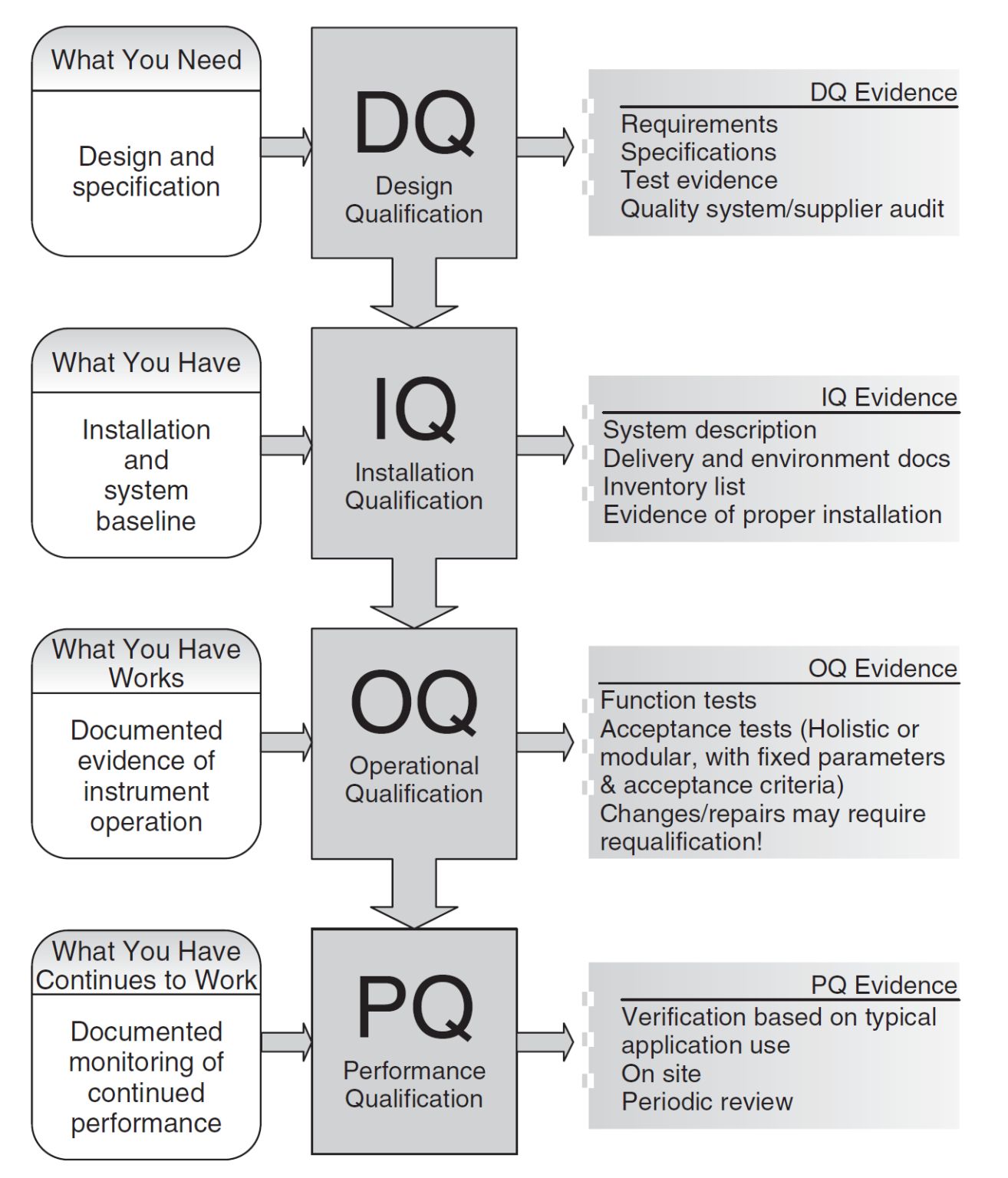

We draft and execute qualification protocols to ensure regulatory compliance of equipment and installations:
We assist our clients in the reception of new equipment: